Itragerm Itraconazole, the broad-spectrum antifungal now in a new improved pharmaceutical form
Itragerm 50 mg is the first low-dose itraconazole on the market, and thanks to its SUBA drug administration technology, it improves the bioavailability of its soluble substances, increasing the drug’s absorption and reliability1.
1 capsule of Itragerm 50 mg is equivalent to one conventional Itraconazole 100 mg capsule
Active ingredient The first low-dose itraconazole on the market
The SUBA technology soluble microparticles contained in Itragerm 50 mg improve bioavailability. Thanks to this innovative new technology, Itragerm 50 mg produces the same therapeutic effect as conventional 100 mg itraconazole at lower doses.
More absorption Better plasma bioavailability than conventional Itraconazole
Thanks to SUBA technology, Itragerm 50 mg improves the plasma bioavailability of itraconazole by 80-90% versus 55% of the conventional formulation.
In Itragerm, absorption is not affected by the gastric pH, so patients who are taking proton-pump inhibitors can now continue to do so.
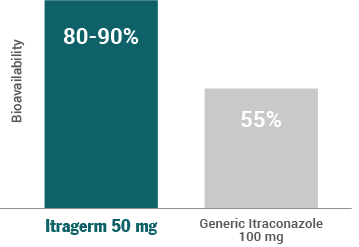
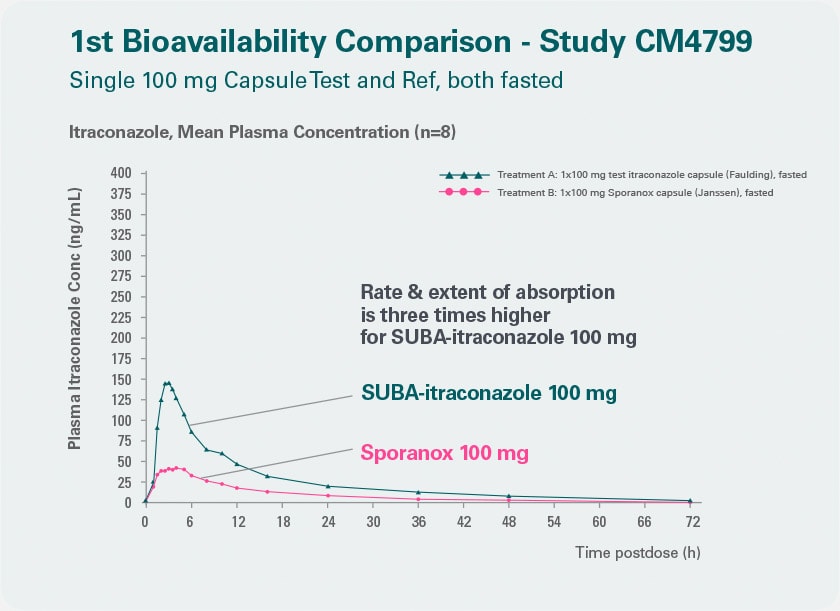
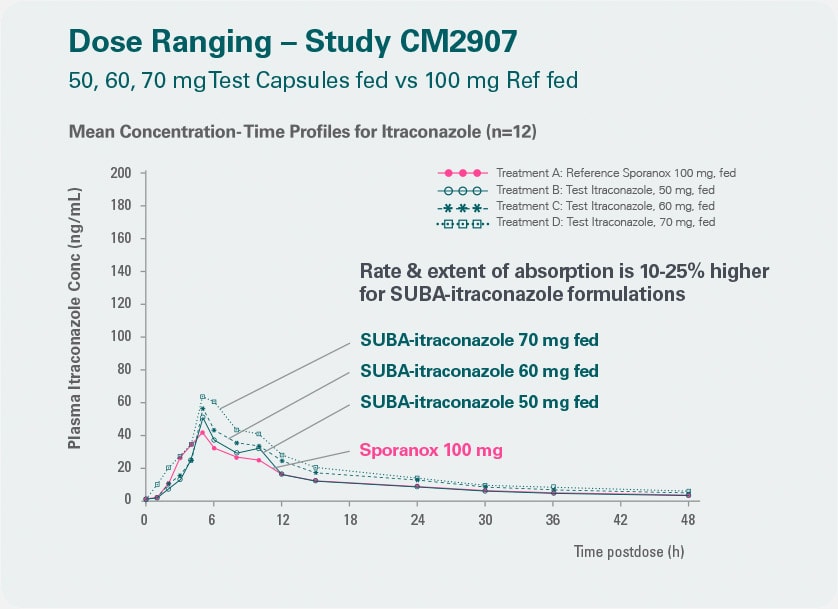
More reliability Better bioavailability in plasma than conventional itraconazole.
The SUBA technology used in Itragerm 50 mg improves itraconazole bioavailability in plasma to 80-90%, compared to 55% for conventional formulations.
Itragerm absorption is not affected by gastric pH. This means that, from now on, patients taking proton pump inhibitors can continue to do so. 1. Mudge S, Hayes D, Ellis D. Mayne Pharma International Pty Ltd, School of Molecular & Biomedical Science, University of Adelaide.

More convenience Easy to administer, easy to take
With Itragerm, patients are not subject to meal times, as it can even be administered on an empty stomach. Also, as it is a smaller capsule, it is easier to swallow. These characteristics can favour adherence to treatment and therefore have a favourable effect on its clinical efficacy. Furthermore, it is the only itraconazole compatible with antacids.

The same posology as conventional Itraconazole
Itragerm 50 mg capsules are for oral administration and can be taken with or without food. One hard capsule of Itragerm 50 mg is equivalent to one hard capsule of conventional Itraconazole 100 mg. Therefore, the recommended dose of Itragerm is half the recommended dose of conventional Itraconazole hard capsules. The posology of Itragerm in adults for each indication is as follows:
Superficial mycosis (of the skin, mucous membranes, eyes)

The bioavailability of itraconazole can be reduced in some immunodepressed patients, e.g. with neutropenia, AIDS or transplants. Double the dose could be indicated.
References:: *Associated with lower absorption variability. 1. Midge S, Hayes D, Ellis D. Single-dose Phase 1 studies to evaluate the more predictable pharmacokinetics of SUBA®-itraconazole, a novel capsule formulation, relative to a conventional itraconazole capsule formulation [Poster]. Australian Society for infectious Disease; Adelaide, 2014.